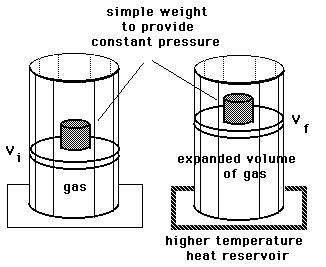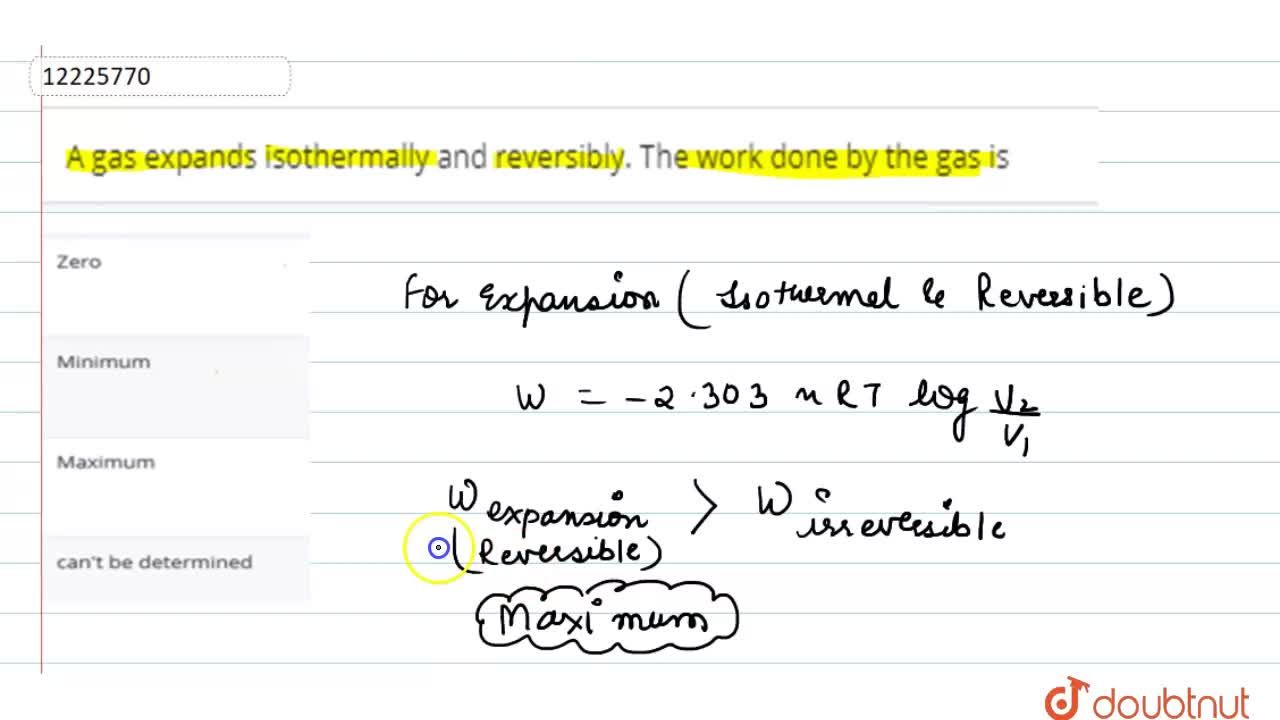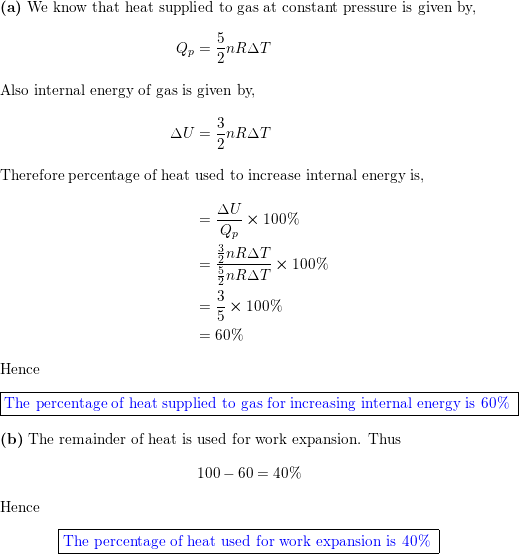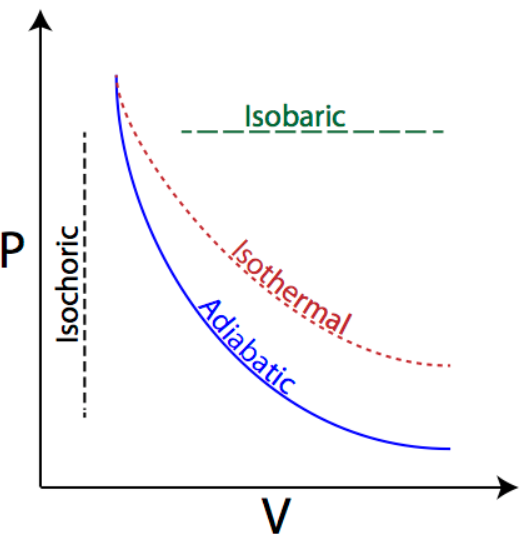
A sample of gas expands from volume ${V_1}$ to ${V_2}$ . The amount of work done by the gas is greatest when the expansion is:(A) Isothermal(B) Isobaric(C) Adiabatic(D) Equal in all cases
A gas expands adiabatically at constant pressure such that its temperature Tprop(1)/(sqrt(V)) , the value of C(P)//C(V) of gas is

An ideal gas expands from initial volume v1 to final v2 in two ways slowly and quickly : Ans is 3.. - Brainly.in

Free expansion of a thermally isolated ideal gas. When the barrier is... | Download Scientific Diagram

An ideal gas expands isothermally from volume `V_(1)` to `V_(2)` and is then compressed to original - YouTube
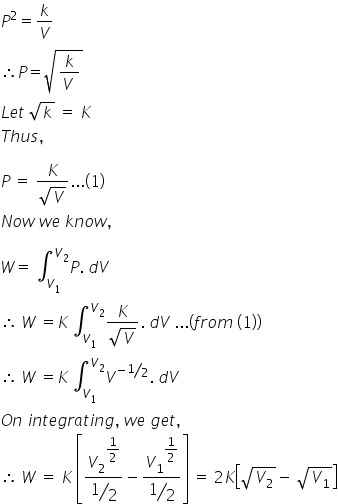
an ideal gas expands according to the law p 2v constant the internal energy of the gas 1 increase continuously 2 decrease continuously 3 remains const gbikc866 -Physics - TopperLearning.com

A gas expands from I to F in the figure below. The energy added to the gas by heat is 438 J when the gas goes from I to F along the

SOLVED: A gas expands against a variable opposing pressure given by P = 10/V atm, where V is the volume of the gas at each stage of expansion. Further, in expanding from