Dapagliflozin as an Adjunct Therapy to Insulin in Patients with Type 1 Diabetes Mellitus: Efficacy and Safety of this Combination – touchENDOCRINOLOGY
Typ-2-Diabetes und Herz-Kreislauf-Risiko: Dapagliflozin hat positive Effekte auch in der kardiovaskulären Primärprävention
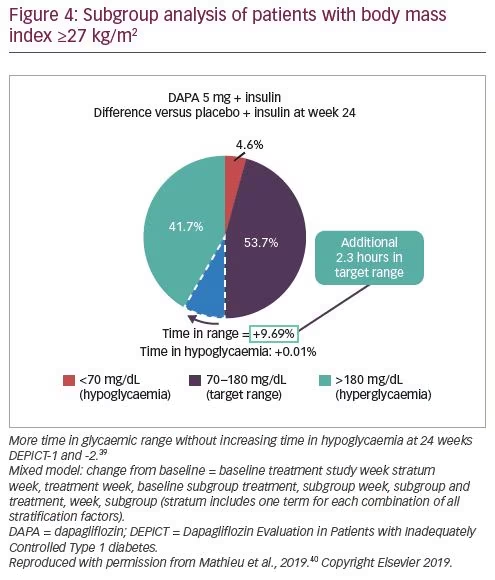
Dapagliflozin as an Adjunct Therapy to Insulin in Patients with Type 1 Diabetes Mellitus: Efficacy and Safety of this Combination – touchENDOCRINOLOGY

Exploring the potential of dapagliflozin in type 1 diabetes: phase 2a pilot study - Media Centre | EASD

Effect of dapagliflozin as an adjunct to insulin over 52 weeks in individuals with type 1 diabetes: post-hoc renal analysis of the DEPICT randomised controlled trials - The Lancet Diabetes & Endocrinology

Dapagliflozin promotes beta cell regeneration by inducing pancreatic endocrine cell phenotype conversion in type 2 diabetic mice - ScienceDirect

Our response to the withdrawal of dapagliflozin (Forxiga) for type 1 diabetes - JDRF, the type 1 diabetes charity
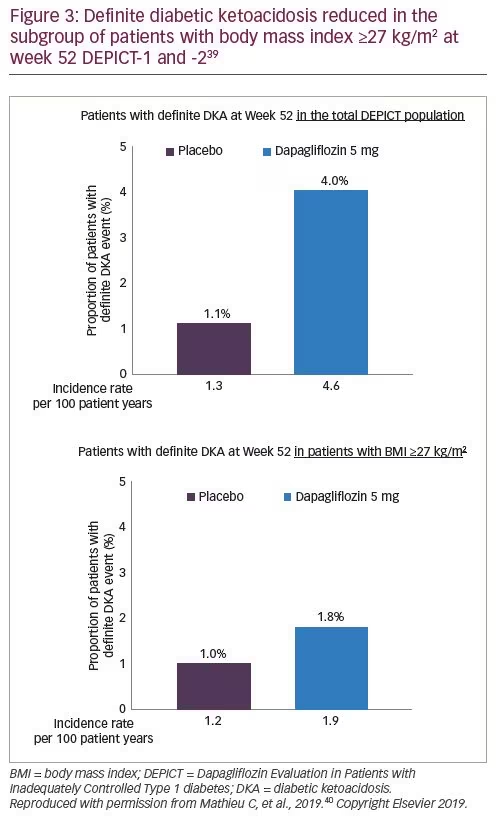
Dapagliflozin as an Adjunct Therapy to Insulin in Patients with Type 1 Diabetes Mellitus: Efficacy and Safety of this Combination – touchENDOCRINOLOGY

Zulassungsänderung - Keine Anwendung von Dapagliflozin bei Typ-1-Diabetes mellitus • diabetologie-online
Wichtige Sicherheitsinformationen zum Risiko einer Diabetischen Ketoazidose (DKA) unter Forxiga 5 mg (Dapagliflozin) zur Anwen

Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study - The Lancet Diabetes & Endocrinology

The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial -